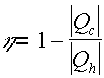Carnot's Theorem
Carnot's theorem states "no real engine operating between two energy reservoirs can be more efficient than a Carnot engine operating between the same two reservoirs" (Serway) This is saying that the efficiency of an actual engine can never be 100% since there will always be an 'exhaust' portion on the engine. All of the work (heat) that is put into the engine can never all be made into usable energy, some of it will be exhausted out, or to the cold reservoir.
The amount of work done by the system can be found using the following conservation of energy
![]()
The thermal efficiency of the Carnot engine can be found using the following, with n being the thermal efficiency:
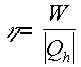
Using the equation of work form above we can find the efficiency of the cycle can be found by the following method: